Periodontitis-Regeneration Modeling
Impact
Periodontitis (PD) is a chronic osteolytic inflammatory disease that results in destruction of tooth-supporting structures and ultimately edentulism. It affects almost 50% of the population and represents a risk factor for numerous systemic conditions with underlying low-grade inflammation 1-3.

Ligature-Biofilm Model Components
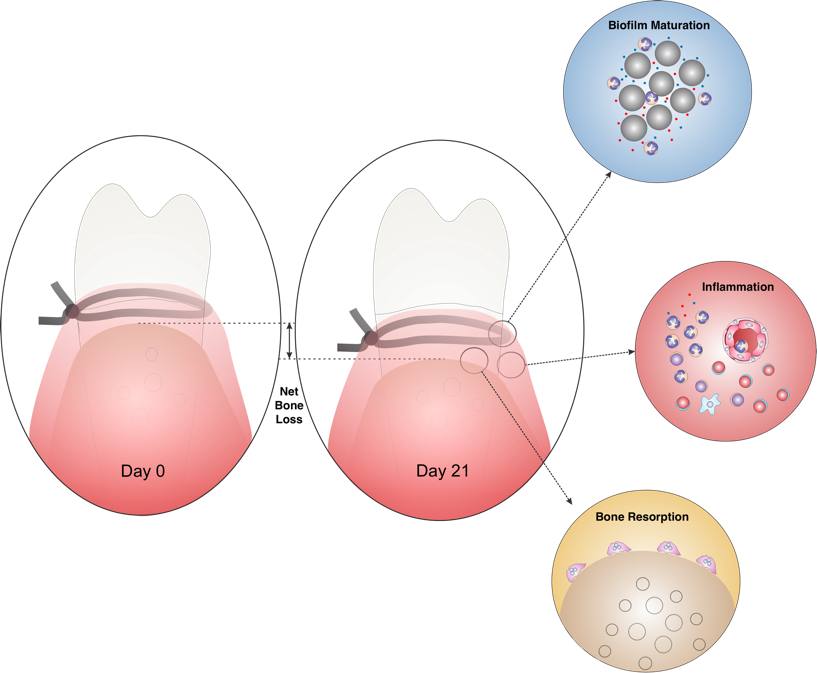
Silk ligatures placed inside gingival sulci of molars serve as biofilm-retentive milieu mimicking calculus in humans. Inflammation-mediated alveolar bone loss that occurs within 2-3 weeks is driven by increased osteoclastic activity.

Introduction
The lack of longitudinal studies in humans linking the transition from gingivitis (GI) to periodontitis (PD) in susceptible individuals or a direct causal relationship between certain bacteria and the onset of bone loss – the hallmark of PD, limits our current understanding of the complex pathogenic mechanisms of this inflammatory disease 4.
Silk ligatures placed in gingival sulci of maxillary molars (M) in mice are retentive for inflammation-inducing subgingival biofilms. They have been used in larger rodents, dogs and primates but to a lesser extent in mice due to the very limited access to their oral cavity 5.
Placement of a small diameter (30 µm) silk sling ligature around maxillary left molars generates a biofilm retentive milieu, sits passively in the gingival sulci and advances apically as disease progresses, which best mimics the advancement of calculus (the ligature in this model) and plaque subgingivally as the supporting tissues are lost. The current protocol uses a mouse model of silk-biofilm induced PD to study inflammatory cell infiltration, gene expression and phenotypic markers of immune cells in the onset and resolution of periodontal inflammation.
Ligature-Biofilm Induced PD
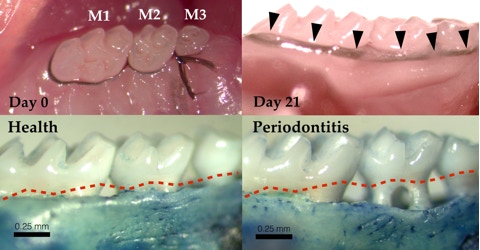
Continuous crossed silk sutures are placed in gingival sulci around maxillary molars (M) and tied between M2 and M3 on the palatal side on day 0. By day 21, ligatures had advanced apically inside gingival sulci (arrows). Methylene blue-stained defleshed maxillae show significant inflammation-mediated alveolar bone loss (iABL) around ligated molars compared to healthy contralateral molars. iABL is measured form the cement-enamel junction (dashed red line) to the alveolar bone crest. Ligatures removed on day 21 and assessed for bacterial colonization reveal approximately equal amounts of aerobic and anaerobic bacteria accumulated around silk filaments.

Cellular Responses to Subgingival Challenge
A critical component of periodontal inflammation that leads to ABL is the cellular response to persistent subgingival microbial challenge. Established PD lesions are characterized by predominant lymphocytic/plasma cell infiltrates but functional defects in the front line polymorphonuclear neutrophils (PMNs) are associated with high incidence and severity of PD 7,8. The roles of PMN in unresolved periodontal inflammation is incompletely understood. To assess cellular responses to silk-biofilm challenge histological sections of ligated molars were stained with Hoechst (stains DNA), FITC-anti-Ly6G (PMN-specific marker) and TRAcP (osteoclast marker).
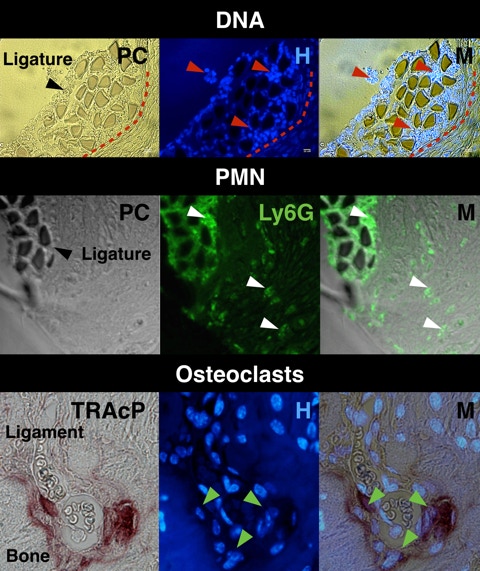
Coronal sections of ligated molars were stained with Hoechst for eukaryotic and prokaryotic DNA. Significant numbers of cells aggregated around silk filaments (red arrows).
Polymorphonuclear neutrophil (PMN) infiltration was assessed by immunofluorescence for PMN-specific antigen Ly6G. Multiple PMN were observed around silk filaments and inside periodontal soft tissues (white arrows).
Osteoclast coverage of the bone-ligament interface was assessed by TRAcP-Hoechst staining. Multinucleated osteoclast were observed along the interface (green arrows).
PC, phase contrast; H, Hoechst; M, merged

Periodontal Tissue Destruction
The hallmark of PD is ABL. However, the destruction of soft tissues always accompanies bone resorption resulting in loss of tooth attachment to bone, periodontal pocket formation and ultimately tooth loss. Whether soft tissue degradation precedes bone loss remains unclear. Loss of tooth attachment measured histologically from the cement-enamel junction (CEJ) to the alveolar bone crest (ABC) can be assessed on Masson trichrome stained coronal sections of ligated molars. Sharpey’s collagen fibers anchor the periodontal ligament (PDL) to bone to ensure tooth attachment to the bony socket. Degradation of Sharpey’s fibers was assessed on picrosirius red stained coronal sections of ligated molars. Additional markers can be used to investigate the loss of PDL.
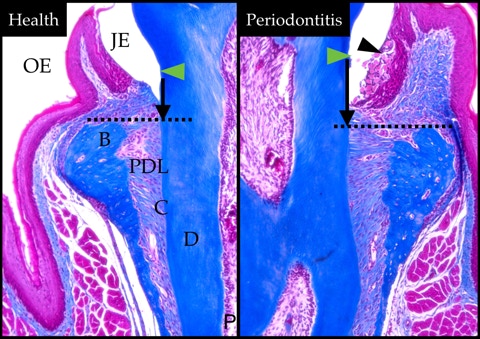
Masson trichrome stained histological sections of molars with PD (right) and healthy contralateral molars (left) show significant loss of tooth attachment (black vertical arrows) from the cement-enamel junction (green arrows) to ABC (dashed line). Black arrow, ligature.
OE, oral epithelium; JE, junctional epithelium; B, bone; C, cementum; PDL, periodontal ligament; D, dentin; P, dental pulp.
Periodontal Tissue Destruction
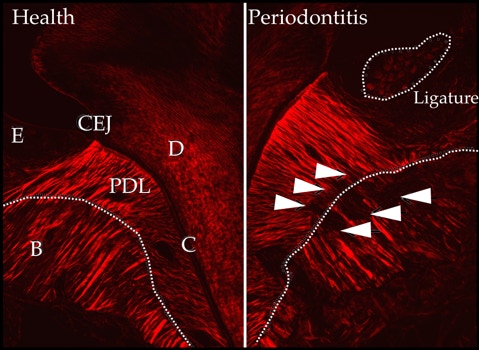
Degradation of collagen Sharpey’s fibers was assessed by using picrosirius red collagen staining of coronal sections. Significant disruption of collagen bundle continuity was observed along the bone-PDL interface (white arrows) around the ligated (right) compared to the healthy molar (left). Loss of collagen bundle integrity occurs in relative proximity to the stimulus (ligature).
E, epithelium; B, bone; C, cementum; PDL, periodontal ligament; D, dentin; CEJ, cement-enamel junction.

Alveolar Bone Regeneration
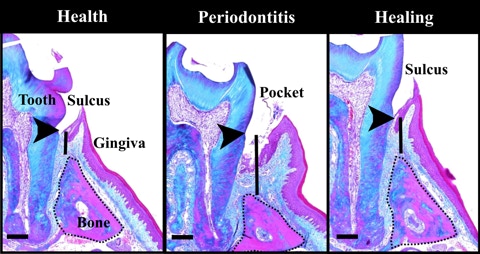
Alveolar bone resorption associated with PD is irreversible. In certain circumstances however, regeneration of lost bone and attachment apparatus can be achieved through guided tissue regeneration procedures or the use of biologics 9-12. This is possible in defects that allow for space maintenance and prevention of fast growing cells, such as epithelial cells, from occupying the space for bone, ligament and cementum regeneration. In non-susceptible mice, bone regeneration following removal of subgingival challenges occurs even in the absence of additional pro-regeneration intervention.
To understand the mechanisms of alveolar bone regeneration mice with ligature-biofilm induced PD were assessed for bone healing and regeneration after removal of stimulus (ligature). Critical time points for bone uncoupling resulting in net bone loss and bone re-coupling resulting in bone gain were established. Pro-resolving activation of macrophages are critical to coupling of bone resorption and formation for regeneration of lost bone due to periodontitis 13.
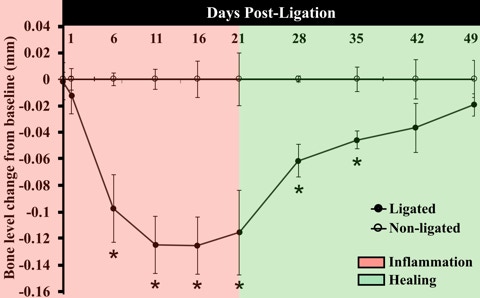
To investigate the course of bone loss and regeneration ligatures were placed on day 0 in 45 wild type mice (C57BL/6J) and 5 mice per group were scarified at each time point: 1, 6, 11, 16, 21 days after ligature 13. In 20 mice ligatures were removed on day 21 and mice were sacrificed on days 28, 35, 42 and 49. Alveolar bone loss was measured by morphometry from CEJ to ABC. Significant bone loss and regeneration occur at day 6 and 42 respectively. * p<0.05. Paired t-tests per time-point.
Bone loss and gain in inflammation (day 21) and healing (day 42)(solid line) were measured as the distance between the cement-enamel junction (arrowheads) and alveolar bone (dotted line) crest on Masson’s trichrome–stained frontal sections.
Scale bar, 0.2 mm.

Heritability of Inflammation-Mediated Alveolar Bone Loss
Existing evidence from studies in identical twins and genome-wide association studies
suggests that heritability of PD in a population can range from 7-50% 14-18. Several gene polymorphisms are thought to contribute to PD predisposition by impacting the recognition and clearance of bacteria, tissue destruction and healing, including genes transcribing interleukin (IL)-1, IL-6, IL-4, IL-10, vitamin D receptor and CD1419.
The links between host predisposition and oral microbiome pathogenic shifts is in its infancy. The major advantage of mouse models of PD is the availability of genetic reference populations (GRP) used to gain insight into host predisposition to iABL. Quantitative trait locus (QTL) genomics in mouse GRPs is a powerful tool for identifying genes involved in biological processes underlying host-biofilm disequilibria.
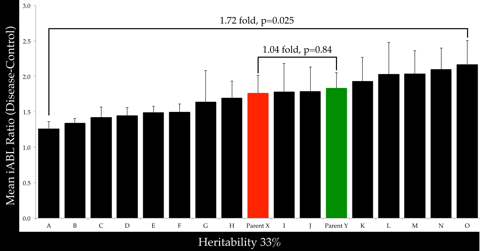
Ligature-biofilm model was used in a panel of recombinant inbred mouse strains (17 strains, N=76) to assess heritability of iABL. 1.72-fold iABL variation was observed between the lowest and highest responders (p=0.025). The continuous distribution of iABL among strains suggest polygenic inheritance of this trait. Heritability of iABL, defined as the ratio of within-strain variance over the total variance, was 0.33. The strain distribution values for iABL were analyzed for genotypic linkage using WebQTL. Results show a linkage with a suggestive QTL on chromosome 2 containing several genes involved in macrophage and osteoblast function.

References
1. Eke PI et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91, 914–920 (2012).
2. Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol 7, 479–480 (2010).
3. Tonetti, MS et al. Treatment of Periodontitis and Endothelial Function. N Engl J Med 356, 911–920 (2007).
4. Bartold PM , Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000 62, 203–217 (2013).
5. Page RC et al. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000 14, 216–248 (1997).
6. Sima C et al. Identification of quantitative trait loci influencing inflammation-mediated alveolar bone loss: insights into polygenic inheritance of host–biofilm disequilibria in periodontitis. J Period Res 51, 237-249 (2016).
7. Oz HS, Puleo DA. Animal Models for Periodontal Disease. Journal of Biomedicine and Biotechnology 2011, 1–8 (2011).
8. Sima C, Glogauer M. Neutrophil Dysfunction and Host Susceptibility to Periodontal Inflammation: Current State of Knowledge. Curr Oral Health Rep 1, 95–103 (2014).
9. Sima C et al. 2014. Rac-Null Leukocytes Are Associated with Increased Inflammation-Mediated Alveolar Bone Loss. Am J Pathol 184:472–482.
10. American Academy of Periodontology. Periodontal regeneration (position paper). J Periodontol 76, 1601–1622 (2005).
11. Needleman IG et al. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev CD001724 (2006).
12. Esposito M et al. Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst Rev 4, CD003875–CD003875 (2009).
13. Viniegra A et al. Resolving Macrophages Counter Osteolysis by Anabolic Actions on Bone Cells. J Dent Res, 97(10), 1160-1169 (2018).
14. Michalowicz BS et al. Periodontal findings in adult twins. J Periodontol 62, 293–299 (1991).
15. Michalowicz BS et al. Periodontal bacteria in adult twins. J Periodontol 70(3), 263-273 (1999).
16. Divaris K. et al. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Human Mol Gen 22, ddt065–2324 (2013).
17. Shusterman A et al. Genotype is an important determinant factor of host susceptibility to periodontitis in the Collaborative Cross and inbred mouse populations. BMC Genetics 14, 68 (2013).
18. Miley DD et al. Heritability of alveolar bone loss from periodontal disease in a baboon population: a pilot study. J Periodontol 82, 575–580 (2011).
19. Laine ML, Loos BG, Crielaard W. Gene Polymorphisms in Chronic Periodontitis. International Journal of Dentistry 1–22 (2010).
Copyright © 2021 Corneliu Sima
All rights reserved.