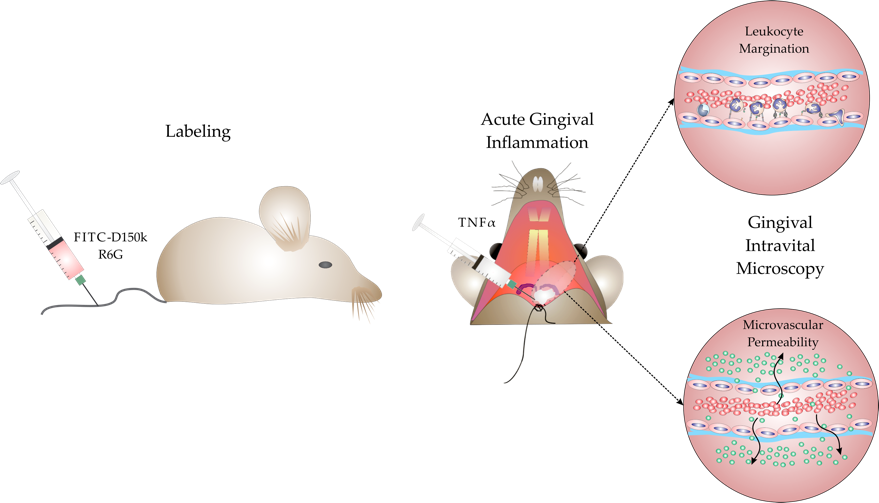
Gingivitis Modeling - Intravital Microscopy
Impact

Gingivitis progresses to and represents an independent risk factor for periodontitis (PD) in susceptible individuals 1-3. Although gingivitis always precedes PD, in some individuals subclinical signs of gingivitis cannot be detected with the current diagnostic tools. Research in this area is still being conducted to develop reliable predictive tools for progression of gingivitis to PD. Intravital imaging is a powerful technique used to get insights into leukocyte behavior in vivo during onset and resolution of inflammation.
Intravital Microscopy in Acute Gingival Inflammation
TNFα injected in the mandibular buccal gingival between incisors acts as pro-inflammatory stimulus that triggers leukocyte infiltration and gingival inflammation. Labeled leukocytes are tracked from intravascular to extravascular compartment by intravital microscopy.

Introduction
In vivo examination of leukocyte behavior in post-capillary venules is essential for understanding the pathobiology of conditions associated with abnormal inflammatory responses such as cardiovascular diseases (CAD), cancer, Alzheimer’s disease, inflammatory bowel disease, ischemia–reperfusion injury and PD 4-8. For PD pathogenesis, increasing evidence validates the ecological plaque hypothesis and polymicrobial synergy and dysbiosis theory that account for an altered inflammatory response to accumulating commensal periodontal biofilms as the cause for opportunistic microbial pathogenicity and disease onset.
Genes associated with polymorphonuclear neutrophil (PMN) survival, delayed apoptosis, and PMN recruitment to sites of inflammation were all found to be up-regulated in PMNs that have located to the diseased periodontium. The heterogeneity in PD expression can be in part explained by the multiple rate-limiting steps in PMN function in relation to periodontal biofilms: recruitment, antimicrobial activity, apoptosis and removal from the tissue by macrophages. Gingival intravital microscopy (GIM) in a mouse model of acute gingivitis investigates leukocyte behavior in vivo, during onset and resolution of inflammation.
Gingival Intravital Microscopy in Acute Gingivitis
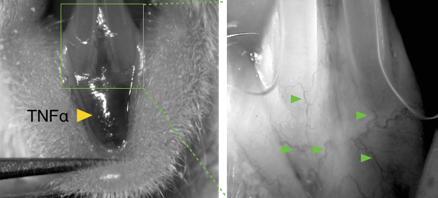
At sites of inflammation TNFα induces increased vascular permeability and upregulation of adhesion molecules in leukocytes and endothelial cells to enhance leukocyte recruitment. Acute gingival inflammation is induced in mandibular incisor region by injection of TNFα in alveolar mucosa on midline. Leukocytes are labeled 2 h after induction of inflammation by IV delivery of rhodamine 6G (R6G) or specific antibodies.
Left: Leukocye-endothelium interactions were assessed in the area coronal to the site of TNFα delivery, between the mandibular incisors.
Right: Several post-capilary venules were selected for microscopic visualization (green arrows).

Leukocyte Margination in Acute Gingivitis
Development of accurate assessment technologies and reproducible methodologies to understand spatial and temporal relationships between blood cells , endothelium and extravascular structures in vivo has increased in recent years. Real-time imaging offers a powerful diagnostic tool for the evaluation of both endothelial cell and leukocyte functions in living organisms.
Fluorescence video-microscopy using leukocyte-labeling dyes or antibodies is being used to evaluate leukocyte behavior in different tissues, including the gingiva 9-15. In humans, gingival angiography has been used to assess vascularization of free gingival grafts when different surgical techniques are used 16-18. Gingival intravital microscopy in mice is useful for getting insights into leukocyte dynamics in onset and resolution of gingivitis.
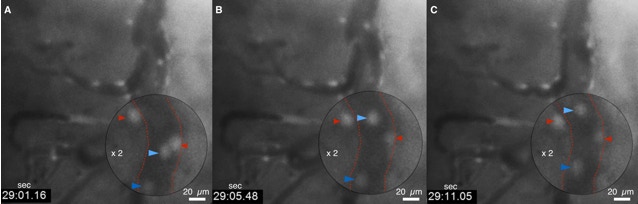
Three time points (A-C) of the same filed of view by wide-filed microscopy showing rolling (blue arrows) and attached leukocytes (red arrows) labeled with R6G.
Assessment of Leukocyte Margination and Tissue Infiltration in the Gingiva
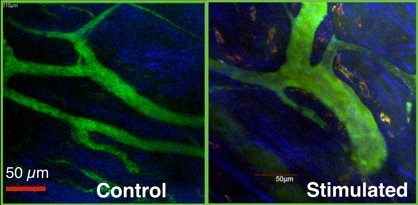
Control
Stimulated
Multi-photon imaging of gingival microvasculature showing recruiting inflammatory monocytes/macrophages (CCR2+) during acute gingivitis induced with TNFα.
Marginating Leukocytes in the Gingiva

Gingival Microvascular Permeability
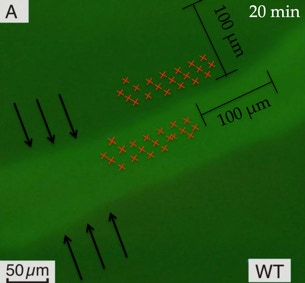
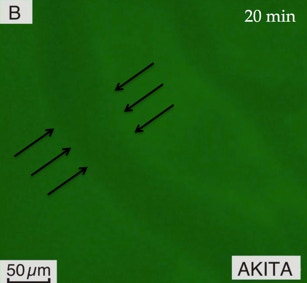
A

One of the earliest events in inflammation is progressive vascular permeability for small molecules and later on for macromolecules, leading to osmotic changes and eventually edema. Using intravital microscopy, changes in microvascular permeability for macromolecules at inflamed sites can be measured by measuring change in fluorescence intensity as the solute (FITC–dextran, Mw 150 kDa) moves across the vessel wall 15, 18, 19. The high Mw (> 2 × albumin) prevents rapid escape of FITC-D from the circulation by extravasation through the vessel wall or by renal clearance, in health.
The sequential intravital images captured within 20-30 minutes from IV delivery, are used to measure microvascular permeability in the gingiva by comparing intravascular (IV) and extravascular (EV) fluorescence intensity. Permeability index (PI) is calculated as follows: PI = EV / IV ~ dIf; EV, extravascular fluorescence intensity; IV, intravascular fluorescence intensity. This formula is derived from PS=JS/SΔC=1/ΔI0 (dIf/dt)i (D/4), where PS is permeability of solute; JS, the flux of solute through vessel; ΔC, the coefficient of diffusion across vessel wall; ΔI0, initial fluorescence intensity of solute filling the vessel; D, vessel diameter; dt, time interval for assessment of permeability; dIf, variation in fluorescence intensity.
Measurements were performed within 20 minutes after IV delivery of FITC-D 150 KDa over 100 µm length of vessel and within 100 µm from vessel wall into extravascular compartment.
A: The FITC–D is retained in the gingival vasculature of a healthy wild type (WT) mouse (light green inside blood vessel).
B: Increased FITC–D extravasation in the gingiva of a diabetic Akita mouse, within the same time interval (light green in the extravascular tissue compartment).
Leukocyte Endothelial Transmigration in the Gingiva
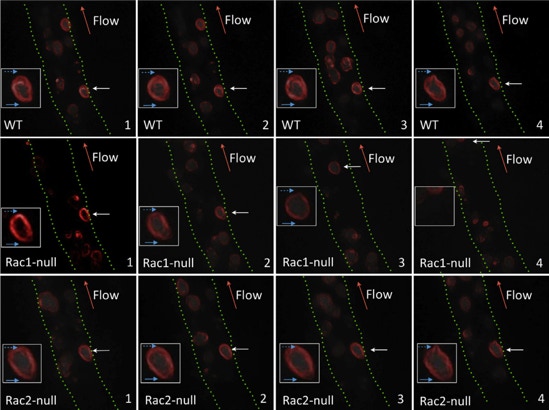
Leukocyte adhesion deficiencies (LADs) represent a group of inherited disorders associated with defects in the expression or function of leukocyte adhesion molecules. Type I-III LADs are caused by deficiencies in structure and/or function of selectins and integrins involved in leukocyte margination and tissue migration, whereas LAD type IV is characterized by deficiencies in Rac2 expression involved neutrophil chemotaxis, margination and superoxide production. LADs are associated with high prevalence of periodontal diseases and tooth loss in primary and permanent dentitions 20.
Snapshot images taken during intravital microscopy at 1-sec intervals for Rac1-null, Rac2-null, and WT mice (each row). Normal attached leukocytes (WT) are able to retract the uropod and project a lamellipodia to migrate through the endothelial wall within the observation period. Rac1-null attached leukocytes were more prone to detach from endothelium before projection of lamellipodia and seemed to fail to retract the uropod. Rac2-null attached leukocytes behave similar to normal leukocytes despite a lower rolling rate along the endothelium. White arrows, leukocyte attached to endothelium; red arrows, direction of blood flow; solid blue arrows, leading edge/lamellipodia; dashed blue arrows, trailing edge/uropod. Insets show magnified leukocyte attached to endothelium during the observation period.

References
Copyright © 2021 Corneliu Sima
All rights reserved.
1. Ranney RR. Discussion: pathogenesis of gingivitis. J Clin Periodontol 13(5):356–9 (1986).
2. Lang NP, Schätzle MA, Löe H. Gingivitis as a risk factor in periodontal disease. J Clin Periodontol 36:3–8 (2009).
3. Califano JV, Research SATCAAOP. Position paper: periodontal diseases of children and adolescents. J Periodontol 74(11):1696–704 (2003).
4. Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol 7, 479–480 (2010).
5. Tonetti MS et al. Treatment of Periodontitis and Endothelial Function. N Engl J Med 356, 911–920 (2007).
6. Jung K. et al . Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J Clin Invest 127(8), 3039-3051 (2017).
7. Bomboi G et al. Alzheimer’s disease and endothelial dysfunction. Neurol Sci 31, 1–8 (2010).
8. Asaduzzaman M et al. P-selectin and P-selectin glycoprotein ligand 1 mediate rolling of activated CD8+ T cells in inflamed colonic venules. J Investig Med 57, 765–768 (2009).
9. Cahalan MD et al. Real-time imaging of lymphocytes in vivo. Curr Opin Immunol 15:372–377 (2003).
10. Chiang EY et al. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods 4:219–222 (2007).
11. Albertine KH, Gee MH. In vivo labeling of neutrophils using a fluorescent cell linker. J Leukoc Biol 59:631–638 (1996).
12. von Andrian UH. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3: 287–300 (1996).
13. Sumen C et al. Intravital microscopy: visualizing immunity in context. Immunity 21:315–329 (2004).
14. Sima C et al. Rac-Null Leukocytes Are Associated with Increased Inflammation-Mediated Alveolar Bone Loss. Am J Pathol 184:472–482 (2014).
15. Sima C et al. Type 1 diabetes predisposes to enhanced gingival leukocyte margination and macromolecule extravasation in vivo. J Periodontal Res 45(6), 748-756 (2010).
16. Busschop J, de Boever J, Schautteet, H. Revascularization of gingival autografts placed on different receptor beds. A fluoroangiographic study. J Clin Periodontol 10(3), 327-332 (1983).
17. Burkhardt R, Lang NP. Coverage of localized gingival recessions: comparison of micro- and macrosurgical techniques. J Clin Periodontol 32(3), 287-293 (2005).
18. Sarelius IH et al. Macromolecule permeability of in situ and excised rodent skeletal muscle arterioles and venules. Am J Physiol Heart Circ Physiol 290:H474–H480 (2006).
19. Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM- 1-mediated signaling. Am J Physiol Heart Circ Physiol 295:H969–H977 (2008).
20. Sima C, Glogauer M. Neutrophil dysfunction and host susceptibility to periodontal inflammation: current state of knowledge. Current Oral Health Reports 1(2), 95-103 (2014).